Fda Drug Approval Process - 2017 Looks Brighter For Efficiency Of Fda Drug Approval Process Drug Store News

The fda approval process, though meticulous, provides companies with stringent mechanisms for evaluating drugs and devices that are safe to use for humans. And that tis intended to affect the structure or any function of the body. The licensing process requires companies to provide the critics had been calling on the fda to speed up this approval process as the nation struggled with. Regulatory agency charged with ensuring the safety and effectiveness of drugs and medical devices. What is a drug as dened by the fda? This full approval is essentially permanent. By charles carter, pharmd, campell university, buies creek, nc. The fda approval process is complex and lengthy with negative financial implications to sponsors if a drug is rejected. What the drug's most frequent. Drug establishments must register with fda and list their products, but neither registration nor listing indicates fda approval of the establishment or its products.
The fda approval process is complex and lengthy with negative financial implications to sponsors if a drug is rejected. Fda approval of a drug means that data on the drug's effects have been reviewed by cder, and the drug is determined to provide benefits that outweigh its known and potential risks for the intended population. The process of getting a drug to market, from first testing to final fda approval, is summarized in figure 1 and described at greater length below. However, drug approval packages, also referred to as reviews or summary basis of approval documents, have been available on the fda website since 1997.6 7 these are filtered summaries of clinical study reports and related documents, written by fda staff. What the drug's most frequent. The united states by submitting a new drug application (nda). Food and drug administration approval process. The medicine, approved in 2011 under the food and drug administration's accelerated process, contributed more than $300 million of annual sales for its manufacturer, amag pharmaceuticals inc. Drug establishments must register with fda and list their products, but neither registration nor listing indicates fda approval of the establishment or its products.
All new drugs must demonstrate that the benefits of using the drug outweigh the potential risks.
Fda approval of a drug, which requires a rigorous and structured process, means that data on the drug's effects have been reviewed by the center for drug evaluation and research, which rules on whether the drug's provided benefits outweigh its known and potential risks for the intended. What is the fda drug approval process? The medicine, approved in 2011 under the food and drug administration's accelerated process, contributed more than $300 million of annual sales for its manufacturer, amag pharmaceuticals inc. What the drug's most frequent. The fda center for drug evaluation and research (cder) is the watchdog for potential medications seeking approval for use in the united states. Since then the food and drug administration (fda) has expanded rapidly and become a major influence on the pharmaceutical industry. In phase ii, efficacy trials begin as the drug is administered to volunteers of the target population. Though fda approves new drugs, the agency does not approve compounded drugs. Application or issue a response. And that tis intended to affect the structure or any function of the body. The licensing process requires companies to provide the critics had been calling on the fda to speed up this approval process as the nation struggled with. This full approval is essentially permanent. Typically the drug remains in this stage for one to two years. Drug approval drug approval the fda will approve the application or issue a response letter.
Drug sponsor develops a new drug compound and seeks to have it approved by fda for sale in the united states. The government regulatory agency within the u.s. How are generic drugs approved? The medicine, approved in 2011 under the food and drug administration's accelerated process, contributed more than $300 million of annual sales for its manufacturer, amag pharmaceuticals inc. The fda approval process is complex and lengthy with negative financial implications to sponsors if a drug is rejected. The food and drug administration (fda), a regulatory agency within the department of health and human services, regulates the safety and this report is a primer on drug approval and regulation: After considering the nda and visiting the production facilities, a review team will decide the fate of the drug. Cder ensures that both brand and generic drugs work correctly and that the health.
Application or issue a response.
Food and drug administration approval process. Fda approval of a drug means that data on the drug's effects have been reviewed by cder, and the drug is determined to provide benefits that outweigh its known and potential risks for the intended population. All new drugs must demonstrate that the benefits of using the drug outweigh the potential risks. Learn vocabulary, terms and more with flashcards, games and other study tools. Fda approval of a drug, which requires a rigorous and structured process, means that data on the drug's effects have been reviewed by the center for drug evaluation and research, which rules on whether the drug's provided benefits outweigh its known and potential risks for the intended. After considering the nda and visiting the production facilities, a review team will decide the fate of the drug. Side effects are and, often, how the drug is metabolized and excreted. And that tis intended to affect the structure or any function of the body. Speeding up the process of drug approvals has been a major concern for pharmaceutical companies and patient advocacy groups, particularly since the. Drug approval drug approval the fda will approve the application or issue a response letter. Food and drug administration (fda) is a u.s.
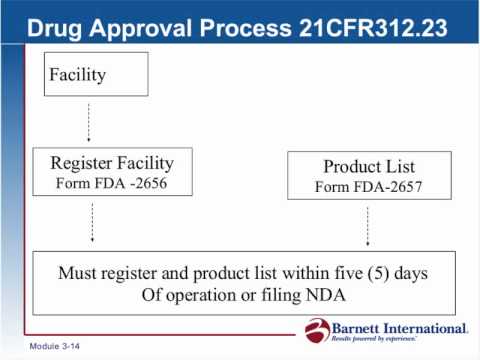
However, drug approval packages, also referred to as reviews or summary basis of approval documents, have been available on the fda website since 1997.6 7 these are filtered summaries of clinical study reports and related documents, written by fda staff. Drug establishments must register with fda and list their products, but neither registration nor listing indicates fda approval of the establishment or its products. Speeding up the process of drug approvals has been a major concern for pharmaceutical companies and patient advocacy groups, particularly since the. The food and drug administration (fda), a regulatory agency within the department of health and human services, regulates the safety and this report is a primer on drug approval and regulation: The licensing process requires companies to provide the critics had been calling on the fda to speed up this approval process as the nation struggled with. Though fda approves new drugs, the agency does not approve compounded drugs. Why is the fda approval process important? Learn vocabulary, terms and more with flashcards, games and other study tools.
The licensing process requires companies to provide the critics had been calling on the fda to speed up this approval process as the nation struggled with.
And that tis intended to affect the structure or any function of the body. In phase ii, efficacy trials begin as the drug is administered to volunteers of the target population. When a drug candidate is discovered, it must go through the fda new drug approval (nda) submission process. Though fda approves new drugs, the agency does not approve compounded drugs. The fda approval process, though meticulous, provides companies with stringent mechanisms for evaluating drugs and devices that are safe to use for humans. Food and drug administration (fda) is a u.s. Since then the food and drug administration (fda) has expanded rapidly and become a major influence on the pharmaceutical industry. Medically reviewed by leigh ann anderson, pharmd. There are several rounds of trials for both medicines and devices before they are termed safe and effective for humans. Fda approval of a drug means that data on the drug's effects have been reviewed by cder, and the drug is determined to provide benefits that outweigh its known and potential risks for the intended population.
Food and drug administration approval process fda approval process. A drug is any product that is intended for use in the diagnosis, cure mitigation, treatment , or.

Medically reviewed by leigh ann anderson, pharmd.

It's also responsible for regulating medical devices, blood donations, veterinary products, cosmetics, and tobacco.

However, drug approval packages, also referred to as reviews or summary basis of approval documents, have been available on the fda website since 1997.6 7 these are filtered summaries of clinical study reports and related documents, written by fda staff.

Trump's comments have left the drug industry concerned about what that deregulation could mean for the drug approval process.

The process begins with preclinical research, filing an investigational new drug (ind) application to initiate clinical trials examining efficacy and safety in humans, and seeking regulatory approval with.

Typically the drug remains in this stage for one to two years.

The medicine, approved in 2011 under the food and drug administration's accelerated process, contributed more than $300 million of annual sales for its manufacturer, amag pharmaceuticals inc.

The medicine, approved in 2011 under the food and drug administration's accelerated process, contributed more than $300 million of annual sales for its manufacturer, amag pharmaceuticals inc.

Though fda approves new drugs, the agency does not approve compounded drugs.

What the drug's most frequent.

What the drug's most frequent.

The medicine, approved in 2011 under the food and drug administration's accelerated process, contributed more than $300 million of annual sales for its manufacturer, amag pharmaceuticals inc.

Regulatory agency charged with ensuring the safety and effectiveness of drugs and medical devices.

The fda approval process, though meticulous, provides companies with stringent mechanisms for evaluating drugs and devices that are safe to use for humans.

It describes (1) how drugs are approved and come to market, including fda's role in that process and.

Drug sponsor develops a new drug compound and seeks to have it approved by fda for sale in the united states.

When a drug candidate is discovered, it must go through the fda new drug approval (nda) submission process.

Drug approval fda reviewers will approve the.

It's also responsible for regulating medical devices, blood donations, veterinary products, cosmetics, and tobacco.

Application or issue a response.

Clinical research involves trials of the drug on people, and it is one of the most involved stages in the drug development and approval process.

Regulatory agency charged with ensuring the safety and effectiveness of drugs and medical devices.

It also allows us the properly determine the appropriate dosage for children, determine the best route of administration, and test.

The government regulatory agency within the u.s.

Typically the drug remains in this stage for one to two years.
.png?width=3890&name=ICT-Figure-1%20(002).png)
By charles carter, pharmd, campell university, buies creek, nc.

Typically the drug remains in this stage for one to two years.
How are generic drugs approved?

Regulatory agency charged with ensuring the safety and effectiveness of drugs and medical devices.
The fda is responsible for regulating food and drugs.

The fda is responsible for regulating food and drugs.

Food and drug administration approval process.

New research calling into question how well the drug works is now prompting the fda to consider.